ATLANTA — With a COVID-19 vaccine in sight, plans are underway on how it will be distributed across the state. Gov. Brian Kemp held a roundtable discussion on Monday with nursing home administrators to talk about the "distribution in the coming weeks."
On Nov. 18, a Georgia Department of Public Health spokeswoman said a 52-page plan has received approval from the CDC, but "there are still many unknowns about the vaccine itself, so it is still considered draft by DPH."
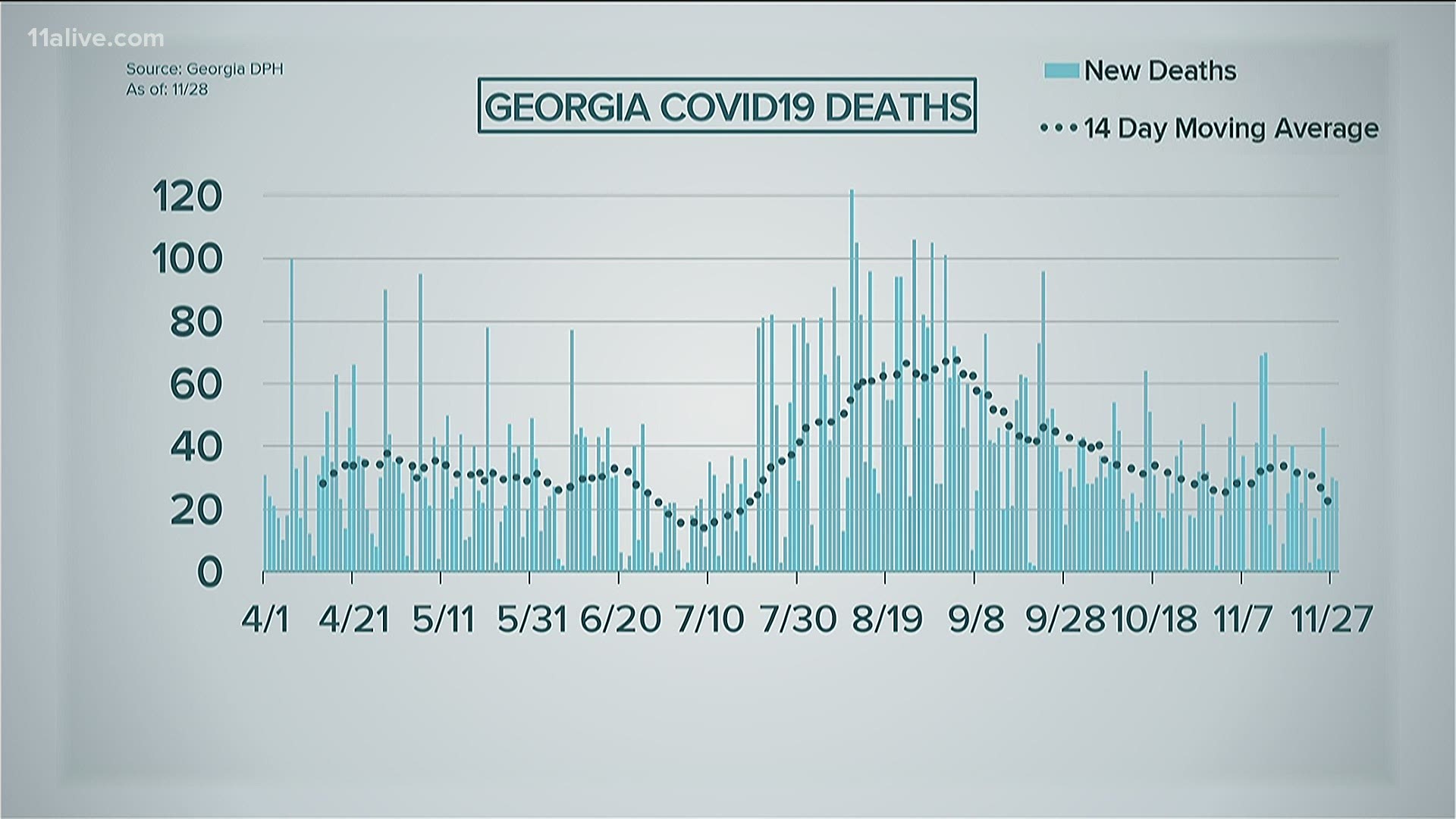
During the roundtable, Kemp said that the current nursing home numbers were still below the summer surge.
There are many factors that come into play, including steps for storing, transporting, and eventually distributing it across Georgia.
The state's plan details four phases for vaccinations:
- Phase one: Healthcare workers, first responders, and people over 60 with multiple underlying conditions making them high risk.
- Phase two: School and childcare workers, essential workers (such as truck drivers, grocery, and food processing workers), homeless shelter and jail staff, anyone 60 and older, and people under 60 with significant underlying conditions.
- Phase three: People 18 to 30 and those in industries with moderate exposure risk (for example hairstylists and restaurant employees).
- Phase four: Anyone else for whom the vaccine is recommended.
According to the CDC, as of Nov. 24, large-scale (Phase 3) clinical trials are in progress or being planned for five COVID-19 vaccines in the United States:
- AstraZeneca’s COVID-19 vaccine
- Janssen’s COVID-19 vaccine
- Moderna’s COVID-19 vaccine
- Novavax’s COVID-19 vaccine
- Pfizer’s COVID-19 vaccine
Already, AstraZeneca, Moderna and Pfizer have said early data shows their vaccines are highly effective -- up to 95 percent.